Animal experimentation ethics committee and authorised body (CEEA/OH)

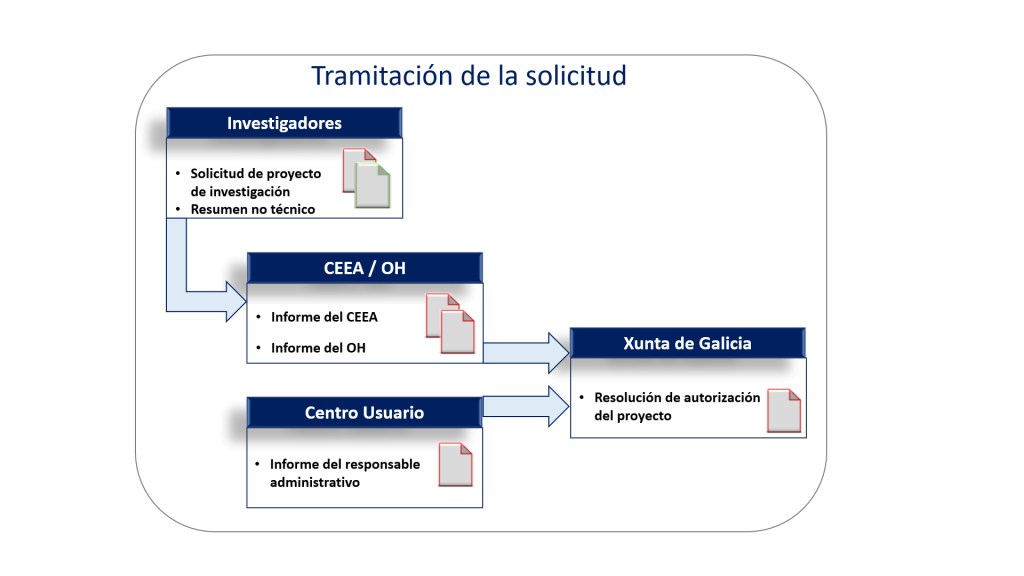
In accordance with the provisions of RD 53/2013, of February 1, which establishes the basic applicable standards for the protection of animals used in experimentation and other scientific purposes, including teaching, the competent authorities must authorise the development of a project involving the use of animals. The Consellería do Medio Rural of the Xunta de Galicia has appointed the IDIS Animal Experimentation Ethics Committee as an Authorised Body for the evaluation of projects requested by centers that use experimental animals.
The Authorised Body (CEEA/OH) of the IDIS will review the animal experimentation procedures, prior to the project application process by the competent authority (Consellería do Medio Rural of the Xunta de Galicia).
Meetings of the Animal Experimentation Ethics Committee and Authorised Body (CEEA/OH)
The members of the CEEA/OH will examine and evaluate all the projects that have been entered in the Committee’s registry up to one week before the meeting date, which is held on the 15th of each month (or the business day after this, if it is a weekend). The dates are estimates and may vary depending on the availability of the members of the Commission.
Download the calendar of the next scheduled meetings here.
Composition
ANIMAL EXPERIMENTATION ETHICS COMMITTEE
- President: María Luz Alonso Alonso. Member of the group C043 – Neuroimaging and Biotechnology.
- Members:
- Francisco Campos Pérez. Leader of the group C042- Translational stroke.
- Clotilde Costa Nogueira. Member of the group C030 – Translational Medical Oncology.
- Natalia Miño Fariña. Professor Faculty of Veterinary. USC.
- Secretary: José Ramón Castro Ruibal. IDIS Research Manager.
AUTHORISED BODY
- President: Antonio González Cantalapiedra. Managing Director of ROF Codina Foundation.
- Members:
- María Luz Alonso Alonso. Member of the group C043 – Neuroimaging and Biotechnology.
- Francisco Campos Pérez. Leader of the group C042- Translational stroke.
- Clotilde Costa Nogueira. Member of the group C030 – Translational Medical Oncology.
- Natalia Miño Fariña. Professor Faculty of Veterinary. USC.
- Secretary: José Ramón Castro Ruibal. IDIS Research Manager.
Contacto
María Luz Alonso Alonso
Forms
Applicable legislation
- Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia
- Ley 6/2013, de 11 de junio, de modificación de la Ley 32/2007, de 7 de noviembre, para el cuidado de los animales, en su explotación, transporte, experimentación y sacrificio
- Real Decreto 1386/2018, de 19 de noviembre, por el que se modifica el Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (Spanish)
Other information of interest
- Search for alternative methods to the use of animals
- Sample size calculation
- The PREPARE Guidelines (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence)
- The ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments)
Application processing
"*" indicates required fields



